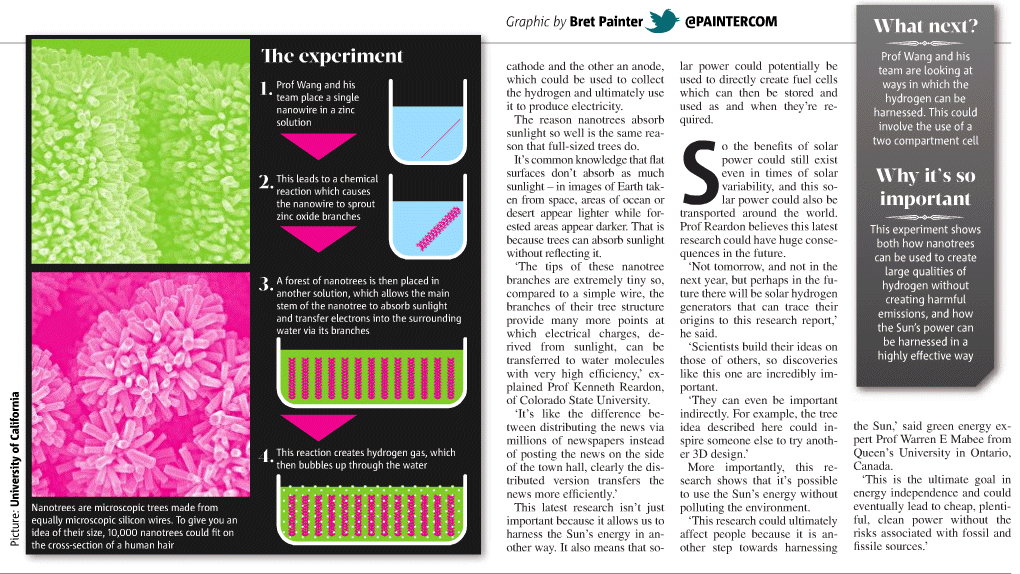
|
 |
New leaf: The promise of artificial photosynthesis
· 16 April 2012 by Nicola Jones
· Magazine issue 2860. Subscribe and save
· For similar stories, visit the Energy and Fuels Topic Guide
It's chemistry's greatest challenge - inventing systems that turn water and sunshine efficiently into cheap, clean energy for all

LOOK at a plant soaking up the sun, and it is hard not to feel a tinge of jealousy. There they sit, day after day, extracting vast amounts of fuel from sunlight, all the while expelling nothing more objectionable than the oxygen we need to breathe. Our fuel-making exploits are ruinously costly and disruptive by comparison: we rip coal, oil and gas from the ground and burn it to produce far more planet-baking carbon dioxide than anyone can use.
What we wouldn't give to mimic the tactics of plants, the true green warriors. "More energy hits the Earth from the sun in 1 hour than mankind uses in an entire year," says Nate Lewis, a chemist at the California Institute of Technology in Pasadena. We know how to turn that energy into electricity - that is what photovoltaic cells are all about. But the sun does not always shine when and where we want it to. Through photosynthesis, plants have the enviable ability to convert sunlight into fuels that they can store now and burn later. If we could do the same, saving solar energy for a rainy day, transporting it to gloomier climes or pumping it straight into a fuel tank, a large part of our energy problems would be solved.
Now megabucks are flowing from, among others, the US government and big energy corporations to try to make that happen. The challenge of artificial photosynthesis is set - but it is proving to be one of the greatest challenges of all.
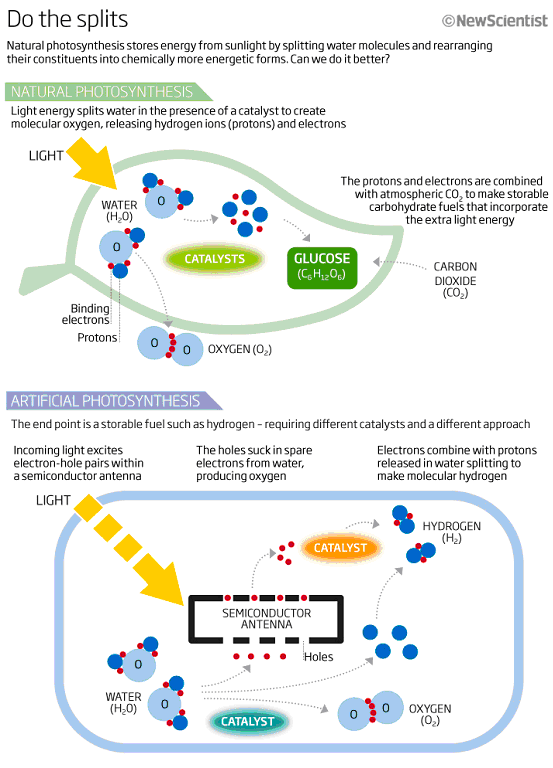
No one said photosynthesis was easy. It took plants millions of years to evolve it, and even now they aren't especially good at it. Photosynthesis is all about using the sun's energy to split water into its constituents, hydrogen and oxygen, and rearranging them into chemically more energetic molecules - in the case of plants, carbohydrates made with the help of atmospheric carbon dioxide. But a standard crop plant stores just a few per cent of the available solar energy in carbohydrates. If the sun shines too intensely its machinery is overwhelmed, shutting down production after about half an hour. The complex natural catalysts that help the process along degrade quickly and must constantly be renewed.
Carbohydrates are not the best storage fuels for our purposes, either. We need something purer, cleaner-burning and with a higher energy density. Hydrogen is a clear choice. It packs a huge punch, storing two-and-a-half times as much energy per kilogram as conventional gasoline. Put it in a fuel cell and you can generate electricity on demand by recombining it with oxygen, with a side product of clean, drinkable water.
All this means artificial photosynthesis is not just about mimicking photosynthesis; it is about doing it better. "It sounds so simple: you're just splitting water," says Daniel Gamelin, a chemist at the University of Washington in Seattle. The devil, though, is in the detail. First you must build an "antenna" akin to a conventional photovoltaic cell to absorb light and use its energy to liberate electrons. Then the chemistry kicks in: those electrons must be guided by catalysts in a complex dance to react with the right molecules to produce the fuels we want.
In 1998, John Turner, working with his colleague Oscar Khaselev at the US National Renewable Energy Laboratory in Golden, Colorado, set the standard. "I was just walking down the hall one day and saw a poster about artificial photosynthesis, and thought - I can help with that," he says. After a year of fiddling with parts, including the solar panels from NASA's Mars rovers, he had his device: a semiconducting chip a few millimetres square that sat in a beaker of dilute battery acid with platinum catalysts. In sunlight, hydrogen gas began bubbling merrily off the chip's surface carrying a full 12 per cent of the incoming sunlight's energy (Science, vol 280, p 425).
There were a few snags, however. The hydrogen bubbled off together with oxygen, a potentially explosive mixture. The device conked out after about 20 hours, as its parts were oxidised and etched away; a gentler system would have fed off water, not battery acid. And it didn't come cheap: at one or two dollars per square centimetre, Turner reckons it was about 10 times too expensive to make hydrogen affordably.
Similar issues have afflicted all artificial photosynthetic systems since. "You've got this three-legged stool: a system has to be efficient, it has to be cheap, and it has to be robust," says Gamelin. Cracking all three at once is the problem, says Lewis. "Pick any two criteria and I can give you that."
The new money aims to get that third leg in place. In 2010, Lewis was signed up to head a new Joint Center for Artificial Photosynthesis based in California, supported by $122 million of Department of Energy funding. That same year, Sun Catalytix, a company born of chemist Dan Nocera's work on artificial photosynthesis at the Massachusetts Institute of Technology, raised $9.5 million from funders including the Indian conglomerate Tata. And scores of other research groups, including Turner's and Gamelin's, are in the game too.
Finicky beasts
The first major task is to find the best material for the antenna. Silicon is relatively cheap and abundant, and absorbs a good chunk of the high-energy photons in the sun's rays, making it the standard for conventional solar cells. But it spits out electrons with an energy of 1.1 electronvolts. Splitting water takes a minimum of 1.23 electronvolts, and in practice needs more to kick-start the reaction.
One way to make up the difference is to stack layers of silicon. Rather like connecting batteries in series, this gives electrons a voltage boost. The latest Sun Catalytix system uses "triple junction" silicon, which works but comes at three times the cost of solar-panel material. Silicon also reacts with oxygen to make an insulating layer of silica, which stops electrons reaching the surface of the antenna where they can be of use. Coating the silicon with an antioxidant can add cost and reduce efficiency. Turner's benchmark device used stacks of gallium arsenide and gallium indium phosphide semiconductors, which absorb energy from different wavelength ranges of light, to multiply the voltage produced, but also suffered from oxidation.
A solution might be to use metal-oxide semiconductors. By their nature these do not oxidise further, which makes them extremely durable, and they are often cheap. But there are billions of combinations of different metals that can make oxides, and finding one with the right properties - that absorbs the right spectrum of light and kicks out enough electrons with the right energy - is not easy. "Oxides are very finicky beasts," says Turner. His team is modelling the likely behaviour of different oxides and have hit on a few good candidates, although they have yet to make them. It is also unclear how well the results will translate to reality, as the models can't handle complications like imperfect crystal structures that are rife in cheaper materials.
Lewis and his team, meanwhile, plan simply to make every oxide they can think of and test them. "He's in the back room mixing things," says Turner. To help hack through the jungle, Lewis's former supervisor Harry Gray has recruited an army of high-school students to make and test their own oxides. They mix up combinations of different metals in different proportions, fire a bank of LED lights at them and watch the voltage and current produced. Gray's team checks samples that look promising, to determine the exact quantities of ingredients and the material's structure. "We've gotten hundreds of potential hits, and something like 20 that are really good looking," he says.
But a good antenna is still only part of the problem solved. Bathed in sunlight, it makes electrons and positively charged "holes" - absences of electrons. Left to themselves, the electrons might simply fall back into the holes and nothing useful would result. To split water, the device needs to line up four holes at one end to suck up electrons from water molecules, producing molecular oxygen and free protons. At the other end, two electrons from the antenna combine with these released protons to form molecular hydrogen (see diagram). Catalysts can smooth these processes, reducing the energy needed to get them going and acting as staging areas for the electrons and holes. Generally, two separate catalysts are needed, one for hydrogen and one for oxygen. Making efficient catalysts as cheaply as possible is another big part of the challenge.
Platinum, used by Turner for both catalysts, works well, but it costs about the same as gold, so the three-legged stool is still missing a leg. Nature has its own imperfect solutions. To make hydrogen, plants use hydrogenase enzymes, which contain a pair of iron atoms to shuffle the electrons. Protein branches around the atoms help the process along by juggling protons. Last year, Monte Helm and colleagues at the Pacific Northwest National Laboratory in Richland, Washington, showed that a similar catalyst with two atoms of nickel, an abundant and cheap element, works much faster than nature's version (Science, vol 333, p 863) - although it hasn't yet been tried out in a photosynthetic system. Simpler compounds, such as molybdenum sulphide, can also do the job. "I wouldn't say anyone has a perfect hydrogen catalyst, but there's a variety of good choices," says Gamelin.
The oxygen-producing catalyst is trickier. Plants use proteins with four manganese atoms, one for each hole involved in the water splitting. But this protein degrades quickly and isn't necessarily the fastest; in the lab, there are better options. Top dog is the highly efficient iridium oxide, but it is heinously expensive. Alternatives based on manganese itself and cobalt are being explored but nothing yet ticks all the boxes.
And that brings us to the really tricky bit of the process. A perfect antenna and perfect catalysts are not enough, says Lewis. "All the parts have to work together and at once." Many catalysts work only in specific pH ranges, so cannot simply be paired up. Pernickety stuff like the nanostructures of the antenna and the catalysts can seriously affect the overall efficiency. Gamelin's team, for example, is currently trying to optimise things by layering a cobalt-phosphate oxygen-producing catalyst onto just the right hole-delivering parts of a large-surface-area antenna. "There's a little bit of voodoo in this process," he says.
Buckets of sunshine
So how close is the voodoo to a workable spell: a realistic artificial photosynthesising system? While there has been no eureka moment, there have been some promising developments. Last year the Sun Catalytix team announced a wireless "artificial leaf" that works, not in battery acid as Turner's did, but in water from Boston's Charles river. It uses catalysts that are relatively efficient and cheap - a blend of nickel, molybdenum and zinc to make hydrogen, and cobalt-borate to spit out the oxygen (Science, vol 334, p 645).
But it is still a stool with legs missing. The device's efficiency is 2.5 per cent, significantly less than Turner's system from over a decade ago, and its components degrade after about a week. The antenna, made of triple-junction silicon, is expensive. The hydrogen it makes comes in at $6 or $7 per kilogram, according to Tom Jarvi, the firm's chief technology officer. At the moment, making hydrogen by re-forming methane costs about $2.50 per kilo.
 Of
course, the company isn't about to reveal ideas in the pipeline, but the
broad aim is to have a system within 10 years that can spit out hydrogen
for less than $3 a kilo at 5 per cent efficiency - with the emphasis on lower
cost at the price of lower efficiency. The ultimate goal is to mass-produce
photosynthesising particles of the sort that can be thrown in a bucket of
dirty water to churn out fuel. "You can shrink your semiconductor down to
a nanoscale and just disperse them in water. That's the best path to low
cost," says Mike Decelle, CEO of Sun Catalytix. It could also mean an energy
revolution in parts of the world far away from electricity grids, where sunlight
is often abundant but access to clean water a problem. If fuel were available
simply by putting a bucket of brackish water out in the sunshine and throwing
in some specks of metallic dust, that could spell the end for paraffin lamps
and the dirty, expensive and cantankerous diesel generators that millions
rely upon to keep lights on and power up other essential services.
Of
course, the company isn't about to reveal ideas in the pipeline, but the
broad aim is to have a system within 10 years that can spit out hydrogen
for less than $3 a kilo at 5 per cent efficiency - with the emphasis on lower
cost at the price of lower efficiency. The ultimate goal is to mass-produce
photosynthesising particles of the sort that can be thrown in a bucket of
dirty water to churn out fuel. "You can shrink your semiconductor down to
a nanoscale and just disperse them in water. That's the best path to low
cost," says Mike Decelle, CEO of Sun Catalytix. It could also mean an energy
revolution in parts of the world far away from electricity grids, where sunlight
is often abundant but access to clean water a problem. If fuel were available
simply by putting a bucket of brackish water out in the sunshine and throwing
in some specks of metallic dust, that could spell the end for paraffin lamps
and the dirty, expensive and cantankerous diesel generators that millions
rely upon to keep lights on and power up other essential services.
"It's definitely really cool," says Gamelin of that idea, and of what Sun Catalytix has demonstrated so far. Even so, Turner guesses it will be at least 15 years before something marketable hits the shelves from any lab. Lewis promises working prototypes within a couple of years, but admits the first ones won't be cheap. Beyond that, he is thinking ahead to a time when the systems don't just make hydrogen but, with a little more chemistry at the back end, a more complex, easier-to-transport fuel such as ethanol - potentially also pulling carbon dioxide out of the air in its production.
That's still a way away. At the moment, says Lewis, "we're like the Wright brothers. Our job is to fail fast and fail frequently and move ahead." How fast failure breeds success will determine how soon we are making fuel as the sun shines.
Nicola Jones is a writer based in Vancouver, Canada
· From issue 2860 of New Scientist magazine, page 28-31.
· As a subscriber, you have unlimited access to our online archive.
· Why not browse past issues of New Scientist magazine?
If you would like to reuse any content from New Scientist, either in print or online, please contact the syndication department first for permission. New Scientist does not own rights to photos, but there are a variety of licensing options available for use of articles and graphics we own the copyright to.
Have your say
Only subscribers may leave comments on this article.
Subscribe now to comment, or link your account to an existing subscription.
More Bang For Your Buck
Thu Apr 12 15:47:43 BST 2012 by Eric Kvaalen
"Carbohydrates are not the best storage fuels for our purposes, either. We need something purer, cleaner-burning and with a higher energy density. Hydrogen is a clear choice."
I disagree. To use hydrogen in vehicles you have to compress it, cool it, or combine it with something else. A liquid hydrocarbon is much to be preferred, and methanol or ethanol can be used with fuel cells (although the efficiency is not very good).
"You can shrink your semiconductor down to a nanoscale and just disperse them in water. That's the best path to low cost," says Mike Decelle.
But if you do that, you get an explosive mixture of hydrogen and oxygen!
login and reply report this comment
More Bang For Your Buck
Fri Apr 13 16:20:07 BST 2012 by Flinty
Unless you use water with a really high biological oxygen demand to use the oxgygen up before it can build up. Combined wastewater treatment/power plants?
login and reply report this comment
view thread
More Bang For Your Buck
Sun Apr 15 07:48:41 BST 2012 by Sandy Henderson
Hi Eric - you covered most of the bases that concerned me when reading this article. The writer failed to say how the researchers planned to separate the oxygen from the hydrogen, and the problems of hydrogen as a commonly used fuel were not addressed. In any case the combination of the most efficient solar cell and the most efficient ( but separate ) electrolysing cell gives an efficiency of circa 30% ,so why not continue improvement of these devices and look to ways to use hydrogen and oxygen to produce synthetic fuels from garbage and sewage ( "resources" that are likely to be always present near large populations?
login and reply report this comment
view thread
More Bang For Your Buck
Mon Apr 16 14:50:13 BST 2012 by E
I agree on all points raised by Eric.
While reading the article, I kept feeling like the scientists were trying to force the shoe to fit the desired results.
The article suggests all the advantages of hydrogen as the only desired end product without any disadvantages, only briefly touching on the entire point of energy research.
What about basic electric current generation and other battery or fuel storage alternatives?
What about the problems with storing a fuel source like hydrogen?
Sure, only needing to convert water would be lovely. But there's a reason we leave handling of significant quantities of hydrogen to explosive experts and rocket scientists.
And why nature didn't select pure hydrogen as the primary fuel source.
For storage, safety, and likely efficiency issues.
login and reply report this comment
More Bang For Your Buck
Mon Apr 16 17:42:15 BST 2012 by ChrisHF
The ultimate goal is to turn sunlight directly into a liquid fuel. We already have lots of ways to turn sunlight (and it's secondary effects) into electricity. Electricity can't be stored without first converting it to something else. We don't need electricity as bad as we need liquid fuels.
login and reply report this comment
More Bang For Your Buck
Tue Apr 17 01:47:24 BST 2012 by Michael Dowling
I wonder if a much simpler system (at least as far as the chemistry is concerned ) might be a better way of storing solar power: use concentrated solar energy to thermally reduce zinc oxide to pure zinc,which could then be transported safely to the end user to produce pure hydrogen by mixing the zinc with 350 degree water.The Israelis were researching this in 2005,and now a more current project is afoot: http://phys.org/news/2012-04-solar-reactor-enable-fuel-derived.html
login and reply report this comment
view thread
More Bang For Your Buck
Tue Apr 17 03:44:38 BST 2012 by Dann
"Carbohydrates are not the best storage fuels for our purposes..."
Maybe not with our current level of technology, but they worked well enough for thousands of years with our old one-horse-power biological engines.